How to Select the Right Pharma Isolators and Containment Systems for Maximum Safety.

Pharma isolators and containment systems are engineered to protect humans, products and businesses while aligning with global GMP standards. However, with numerous configurations and performance grades available, pharma manufacturers often struggle to choose the right system.
In this blog, we have outlined the key points to consider when selecting the correct pharma isolators and containment systems.
Tips to select the right pharma isolator and containment system
Selecting the right isolator or containment system is a technical decision that is tied to process risk, containment level, and compliance requirements. Don’t confuse a cleanroom with a containment system. Cleanrooms protect the product, while isolators protect both the product and the operator, especially when the material is toxic.
Read More: The Importance of Containment Systems in the Pharmaceutical Industry
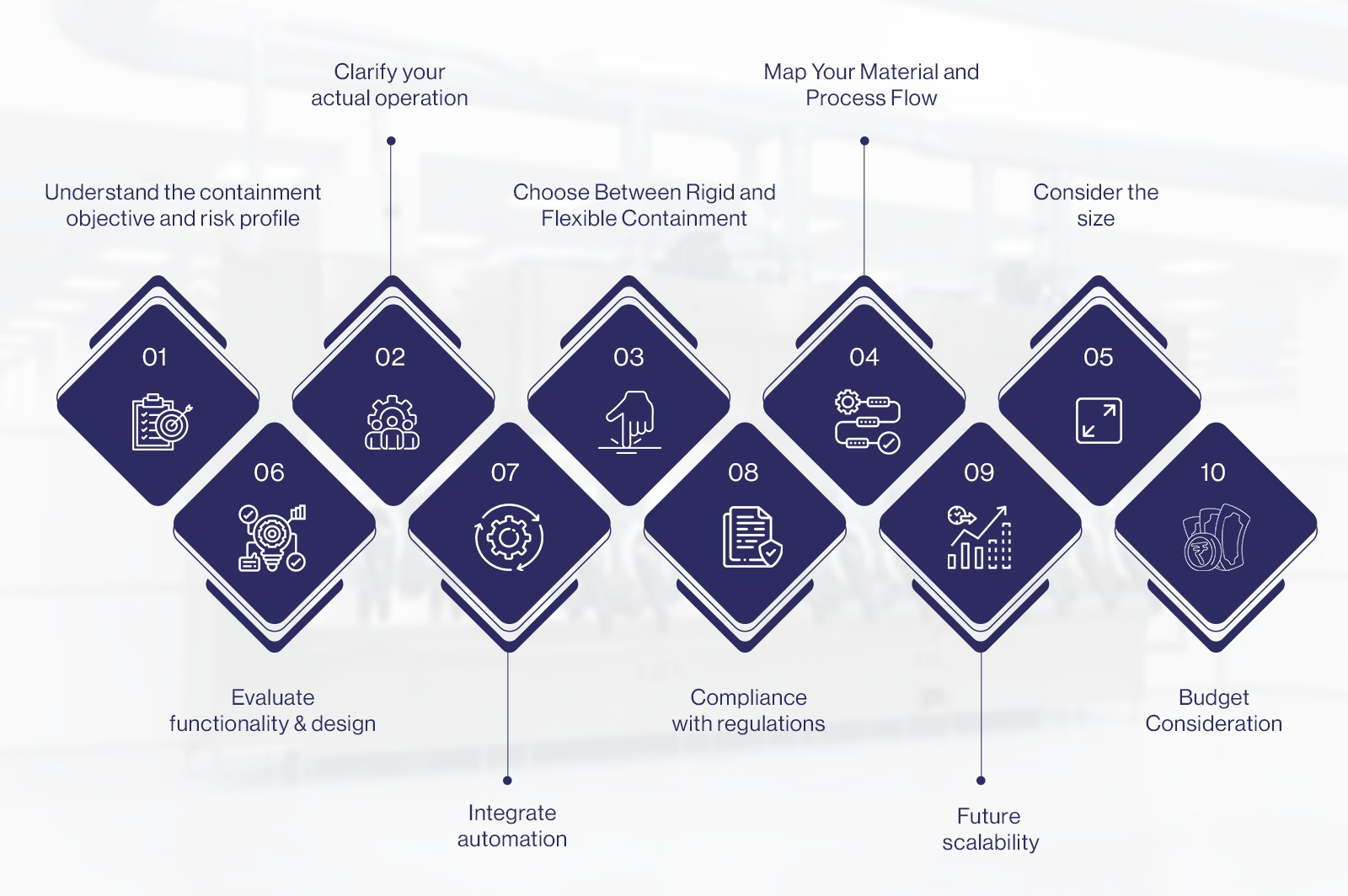
The following factors should be considered when choosing pharma isolator & containment systems for maximum safety:
● Understand the containment objective and risk profile:
Always match your system to the product’s OEL (Occupational Exposure Limit) and OEB (Occupational Exposure Band)
- Product Type & Sterility Levels: Determine if your product is hazardous or sterile, as this defines whether you require a negative pressure, positive pressure, or dual-chamber system. Furthermore, the phase of matter of your product (powder, liquid, or volatile solvent) influences your containment choice.
- Example: HPAPIs (Highly Potent Active Pharmaceutical Ingredients) are often hazardous and require advanced containment systems that limit operator exposure to nanogram levels.
If your product lacks defined OELs, work with a toxicologist to estimate safe exposure limits before equipment selection.
● Clarify your actual operation:
Your choice of pharma containment system depends on the actual operation of the containment. This dictates the isolator design, ergonomics, and pressure configuration.
- Application: Select containment systems according to the tasks being performed.
- Example: Handling powders, aseptic filling, or sterility testing each have unique requirements.
● Choose Between Rigid and Flexible Containment:
Depending on your application, select either a rigid system for high containment or a flexible pharma containment system.
- Rigid isolators: Apt for long-term, high-containment or sterile operations.
- Flexible isolators: Suitable for short-term, small-batch tasks when speed, cost, or simplicity matters, such as sampling, weighing, or dispensing.
● Map Your Material and Process Flow:
Different processes demand different system designs. For engineering an appropriate containment system for your application, it is essential to consider material transfer from one process to another. This is because every transfer or open operation equals a risk of contamination and exposure to the operator.
- Containment review: Understand the process flow and conduct a detailed containment review for each step, from entry to exit. When you map the entire process, you also understand the cleaning requirements between batches or products, allowing you to design your system accordingly.
- Example: If a product exits and re-enters the containment zone, you may need: airlocks, pass-boxes or double-door systems.
● Consider the size: After mapping your requirement, it is essential to design the pharma isolator that aligns with the space constraints of your facility.
- Match isolator size to batch volume and equipment space: Avoid oversized systems that waste space or undersized ones that restrict workflow. Inspect the facility layout, including door clearances, ceiling height, and HVAC access.
- Ensure compatibility with internal equipment: The isolator must accommodate all tools, weighing systems, or containers used inside.
● Evaluate functionality & design:
Select isolators that enhance functionality. Poor design can compromise both the safety and efficiency of pharma isolators.
- Ergonomics: Your containment system should promote a safe and comfortable workflow. For this, extensive tests are performed and a 3D design is prepared before the isolator manufacturing process starts.
- Cleaning: The drainage systems should be designed with a properly sloped floor. Position to ensure complete liquid removal & prevent residue buildup.
- Maintenance access: Critical components, such as filters, control panels, and glove ports, should be accessible for service without requiring the dismantling of major parts.
- High-efficiency filtration: The exhaust system & HEPA filtration systems must be designed to capture potent compounds before releasing air into the environment.
● Integrate automation:
Automation reduces dependency on operators and the risk of human error. The process equipment and the isolator systems must have a standard control interface. Choose your automation level based on the complexity of your process.
- HMI-PLC based control system- Ideal for smaller, standalone systems.
- SCADA-based control system – Apt for large-scale setups that need real-time management of equipment or processes.
● Compliance with regulations:
Choosing a compliant system ensures you’re audit-ready and meet global standards. It is recommended to select a pharma isolator manufacturer who provides compliance-ready systems.
Key validation checks include
- Leak testing
- Air cleanliness testing
- Drainability checks
- Interlocks
- OEL (Occupational Exposure Limit)
- GMP compliance
- Future scalability: If you anticipate process changes or expansions, choose modular enclosures that can be reconfigured or extended without requiring significant redesign or downtime.
- Budget Consideration: Cost is a critical factor while selecting your pharma isolator and containment system. However, it shouldn’t be at the expense of safety or compliance.
Points to consider before finalising the budget:
- Quality
- Regulatory compliance
- Energy use
- Validation cost
- Expected process upgrades
● Recommended Products from F Plus Healthcare Technologies:
As a pharma machinery manufacturer, we recommend our flagship solution engineered for operator and product protection across high-containment and aseptic workflows
Features:
- Dual-pressure (positive/negative), fast cleaning, cross-contamination control
- Can be easily switched between pressure modes as needed.
Material of Construction: SS316L for pharma-grade durability and cleanability
Certified Compliances:
- GAMP 5: for automation and software compliance
- cGMP: Current Good Manufacturing Practices
- CE-marked: European compliance
- ATEX-rated: for safe use in explosive/flammable environments
Read More: Containment System Manufacturer and Solutions with Their Benefits
Conclusion:
Your choice of containment equipment for pharma will safeguard the quality of your product, streamline your processes and ensure compliance with international standards. This means you are audit-ready and have a clear competitive edge in regulated markets.
For more guidance on selecting containment solutions tailored to your operation, you can contact us at [email protected] or on WhatsApp at +91-7506217809 / +91-8655163818

